* Professor and Department Chair/Head, Dept. of Chemistry and Physics, Mount Saint Vincent University.
* Adjunct Professor at Dalhousie University, Saint Mary’s University, and Université Laval.
* Director of Accreditation & Member of the Board of Directors, Canadian Society for Chemistry (CSC), Chemical Institute of Canada (CIC).
* Member, Commission for Quantum Crystallography – International Union of Crystallography (IUCr).
* Member, Canadian National Committee for Crystallography (CNCC).
Lecture 31: Cherif Matta
ATP Synthase: More than just a biocatalyst?
Chérif F. Matta
Dept. of Chemistry and Physics, Mount Saint Vincent University, Halifax, Nova Scotia,
Canada B3M 2J6, and Dép. de chimie, Université Laval, Québec, Québec, Canada G1V 0A6
Cherif.Matta@msvu.ca
ABSTRACT
New biological functions of ATP synthase have recently been proposed. It is now thought that this enzyme interferes with the energetics of the reaction itself and not just with the potential energy barrier (which determines the rate) of the reaction. This unusual role is due to the enzyme's electrostatic potential.
Five accurate crystallographic structures of ATP synthase were used to compare their electrostatic potentials and electrostatic fields by solving the Poisson-Boltzmann equation in an environment simulating that of the mitochondrion. It is found that the potential difference experiences by a proton due solely to the electrostatic potential of the enzyme itself is within an order of magnitude of the chemiosmotic potential difference (the latter of the order of 200 mV). It is therefore suggested that a new term needs to be added to the ΔG expression of the chemiosmotic theory such that :
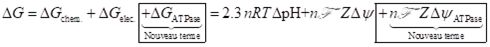
In summary, three different roles can be assigned to ATP synthase:
- Its putative (textbook) role, which is catalysis (lowering the ΔG‡) of the reaction:
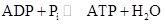
- A novel role, which is the modification of the ΔG of the proton translocation reaction from the intermembrane space to the mitochondrial matrix.
- A second novel role, which is to create a potential barrier regulating the rate of proton translocation through the enzyme (and which has nothing to do with the barrier in reaction (1) that the enzyme catalyzes).
In other words, because of the structure of the enzyme itself, ATP synthase appears to have two secondary roles, at least in the five structures studied.
Time permitting, the great controversy surrounding the operating temperature of mitochondria will be briefly addressed.
REFERENCES
- Vigneau, J.-N.; Fahimi, P., Ebert, M.; Cheng, Y.; Tannahill, C.; Muir, P.; Nguyen-Dang, T.-T.; Matta, C. F., ChemComm 58, 2650-2653 (2022).
- Apps, S., Chemistry World (28 Feb. 2022).
- Fahimi, P.; Matta, C. F., Trends Chem. 4, 96-110 (2022).
- Nasr, M. A.; Dovbeshko, G. I; Bearne, S. L.; El-Badri, N.; Matta, C. F., BioEssays 41, 1900055 (2019).
- Matta, C. F., Massa, L., J. Phys. Chem. A 121, 9131-9135 (2017).


